Abstract

Introduction: Survival rates continue to improve after allogeneic HCT (Gooley et al, NEJM, 2013). Population-based studies also indicate overall improvement in survival of older (60-80 years old) AML patients (pts) (Bower, Blood Cancer Journal, 2016). Yet, only a small minority (6%-8%) of them receive HCT (Medeiros, Ann Hematol. 2015). Given these potentially incongruent findings and the changing face of survival in AML, we designed the first prospective multi-center longitudinal study dating from first presentation of adults with AML to be treated at one of 13 different referral centers that provide both AML treatment and HCT. We compared survival according to whether or not pts received HCT at later time points.
Methods: We enrolled 695 pts (Table 1). Data on demographics, AML status, cytogenetic risks per European Leukemia Network (ELN), and response; age; comorbidities per the HCT-comorbidity index (CI); function including activities of daily living (ADL); frailty; geriatric assessment including cognition; QOL including the Functional Assessment of Cancer Therapy-Bone Marrow Transplant Scale (FACT-BMT), Euro-QOL 5-Dimension scale, ENRICHD Social Support Instrument, Social Activity Log, and Patient Health Questionnaire 9-item Depression Scale (PHQ-9) were collected at enrollment and at 1, 3, 6, 9, 12, 18, and 24 months thereafter.
We used time-dependent Cox regression analyses to identify baseline and time-dependent risk factors associated with mortality in the overall population. The factors identified as significantly associated with mortality (p<0.05) were used to develop multivariate models examining the association between HCT and mortality within 1) the general population as well as those with 2) intermediate vs 3) unfavorable ELN risk, and 4) vulnerable pts (age ≥60 years or HCT-CI scores ≥4). The latter group constituted the majority (76%). In these analyses, all pts were considered to be in the non-HCT group until receipt of HCT at which time they enter the HCT group. The contribution of deaths to the hazard ratio (HR) for HCT reflects the relative number and characteristics of pts remaining at risk in the two groups at the time a death occurs.
Results: Median follow-up was 16.8 months (range 0.1-52.4). In the initial multivariate analyses, the following were identified as significantly associated with an increased risk of mortality (Table 2): HCT-CI scores ≥5 (p<0.0001), age ≥70 years (p<0.0001), intermediate (p=0.03) and high ELN risk (p<0.0001), relapsed/refractory AML at enrollment (p=0.0005), relapse or refractory response to initial treatment after enrollment (p<0.0001), frailty per walk test (p=0.004), impaired QOL per FACT-G scores (p=0.02), increased depression per PHQ-9 (p=0.03), and dependent status per ADL scores <14 (p=0.05).
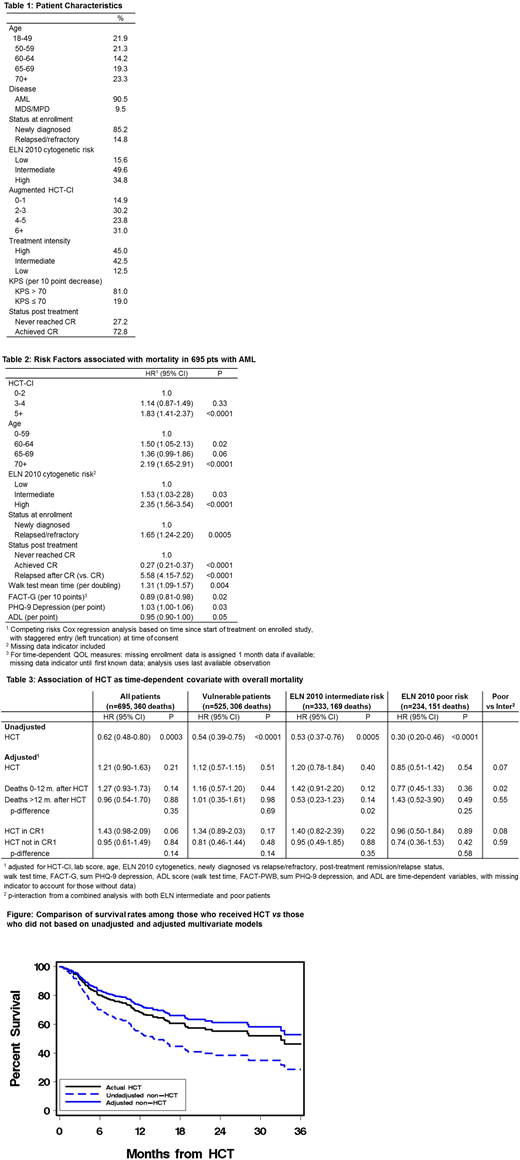
Survival after HCT was 58% at 2-years. Initial unadjusted analyses showed significantly lower risks of mortality in association with receiving allogeneic HCT (p=0.0003). These findings were similar in pts with intermediate (p=0.0005) or unfavorable (p<0.0001) ELN risk and in vulnerable pts (p<0.0001) (Table 3).
However, in the adjusted models, the advantage of HCT in reducing mortality rates was lost both in the overall population (p=0.21, see figure) as well as in the other groups (p>0.54, 0.40, and 0.51, respectively, Table 3). Formal tests of interactions (Table 3) showed no statistically compelling evidence that the association of HCT and mortality varies with respect to the timing of mortality or to the underlying ELN risk.
Conclusions: In a prospective observational study, adjusting for key AML-specific and pt-specific variables negated the observed benefit of HCT over non-HCT therapies in reducing mortality rates among AML pts. Our results might reflect 1) improvement in supportive care and non-HCT therapies, 2) a relatively high non-relapse mortality early after HCT and the need for longer follow-up to demonstrate an adjusted benefit of HCT, and 3) the high selectivity of the transplant eligibility process, as we accounted here for variables that are often ignored in "genetic assignment" randomized studies (i.e. comorbidities and function).
New randomized trials are needed; however, these trials have to be more inclusive of vulnerable pts and measure pt-specific variables. Trials focusing on reducing burden of comorbidities, frailty and poor function are needed alongside trials to treat AML with or without HCT.
Gerds:Celgene: Consultancy; Apexx Oncology: Consultancy; CTI Biopharma: Consultancy; Incyte: Consultancy. Shami:JSK Therapeutics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Baston Biologics Company: Membership on an entity's Board of Directors or advisory committees; Lone Star Biotherapies: Equity Ownership; Pfizer: Consultancy. Rizzieri:Teva: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Wang:Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Novartis: Speakers Bureau; Novartis: Speakers Bureau; Amgen: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Faderl:Jazz Pharmaceuticals: Employment, Equity Ownership. Koprivnikar:Alexion: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Otsuka: Consultancy. Sekeres:Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees. Becker:GlycoMimetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal